Identification of genetic causes of diseases
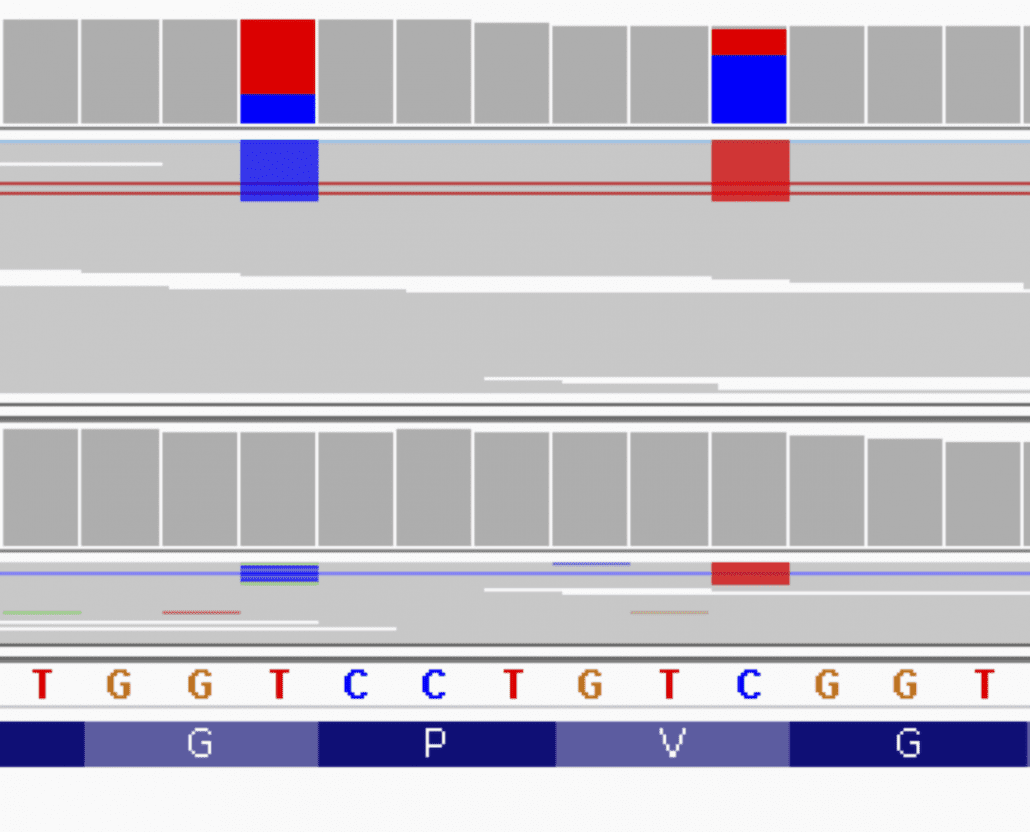
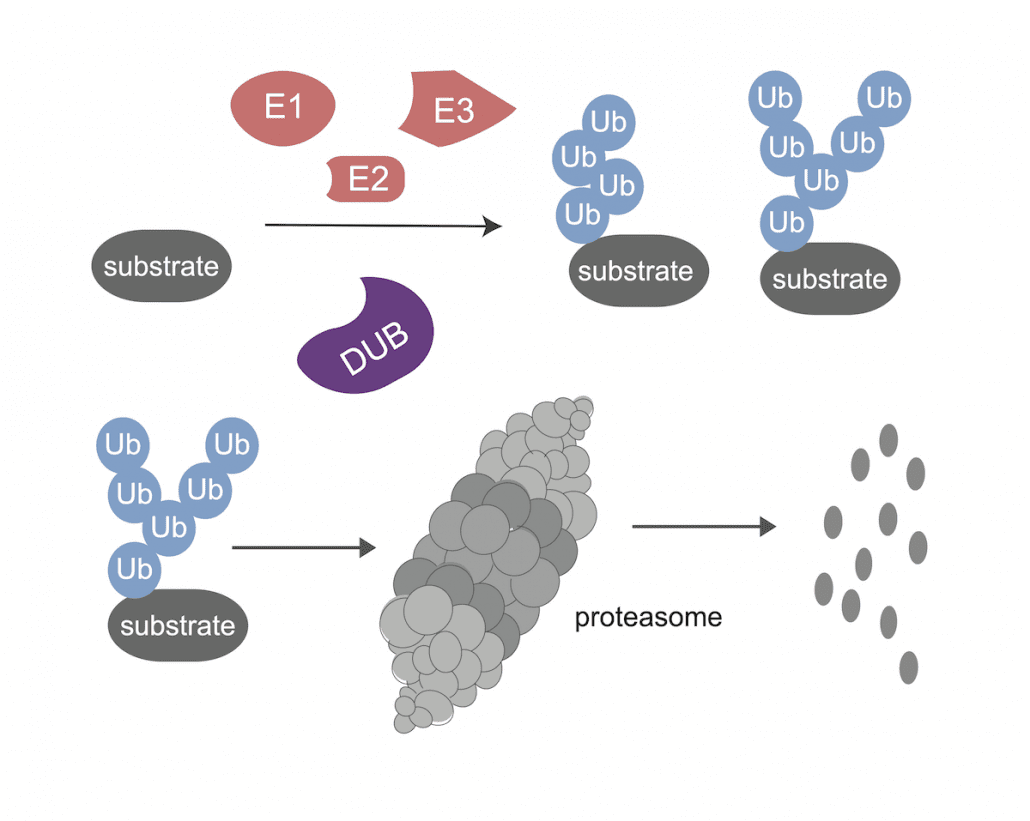
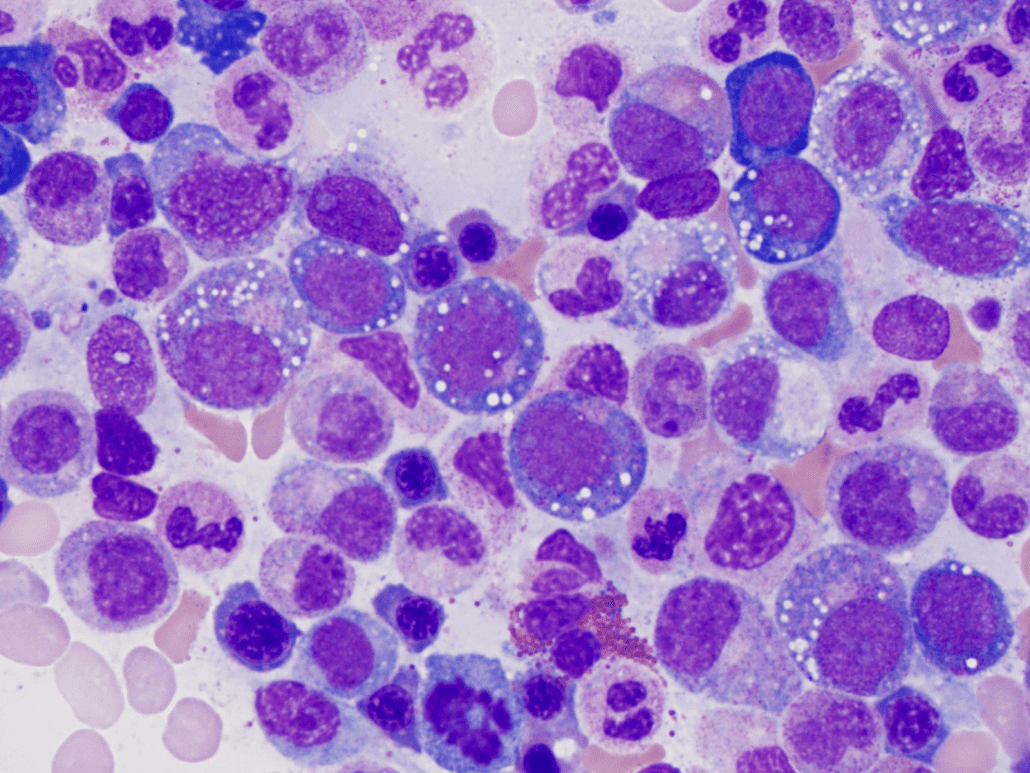
Our group is interested in identifying the genetic causes of autoinflammatory disease using novel sequencing approaches and bioinformatic pipelines. We perform genetic sequencing of patient samples both from our integrated Inflammatory Disease Genetics Program at NYU and from patients referred to us from other centers. This data is analyzed using multiple different genotype-first (Beck DB et al. NEJM 2021), and phenotype-based approaches (Beck DB, Basar MA et al. Science Advances 2021, Beck DB et al. AJHG 2020 b) with the goal of identifying new disease-causing genetic variants. These variants are then modeled in the laboratory to determine how they alter cellular pathways and how these alterations lead to key features of disease. This work has led to the identification of acquired genetic errors of immunity: immune dysregulation caused by cell-type restricted somatic mutations. We are analyzing exome and genome data from patients with suspected acquired errors of immunity and are identifying new variants in UBA1, and other genes, that will lead to additional diagnoses and mechanistic discoveries. Although focused on inflammatory diseases, our work has extended into other areas including developmental diseases, primary immunodeficiencies, and other undiagnosed cases. Our overall goal is to provide genetic diagnoses to facilitate treatment and management in patients and achieve the goal of molecular medicine.